Current lithium batteries are at risk of catching fire if damaged, a problem that the new development does not have. In addition, the battery created by the researchers can be stretched, bent, and even partially destroyed without consequences.
Previous developments in this area could not boast a long service life and a large number of charging cycles. The Berkeley battery overcame these and other limitations, demonstrating its ability to withstand at least 500 charging cycles. Modern mass-produced lithium-ion batteries withstand about the same number of charging cycles.
In the process of creating a battery that is resistant to rough physical impacts, the researchers solved two main problems. First, the battery must not contain toxic materials. Second, the electrolyte structure must be able to maintain its shape on its own - if that definition is appropriate for an electrolyte in the form of "jelly". The scientists accomplished both tasks, although not everything turned out perfectly.
"Modern batteries require a rigid shell, because the electrolyte used in them is explosive. We wanted to create a battery that could be used safely without a rigid package, the scientists explain. — Unfortunately, flexible packaging made of polymers or other elastic materials can easily allow air or water to pass through, which will react with standard electrolytes, generating a lot of heat and potentially causing fires and explosions. That's why in 2017 we started experimenting with quasi-solid hydrogel electrolytes.“
Since there is no ready-made solution, researchers try many compounds before achieving the formation of reliable molecular bonds in the electrolyte, while maintaining acceptable ionic conductivity. In particular, it is now possible to increase the operating voltage of the battery to 3 V and even slightly higher, while previous quasi-solid hydrogel electrolytes limited this indicator to a level of about 1.2 V, which is insufficient for practical use.
The new electrolyte is based on so-called zwitterionic polymers - a class of macromolecules containing both positively and negatively charged groups in the main or side chains. These charges are located close to each other and often neutralize each other, forming molecules with overall electrical neutrality. In batteries, this polymer uses its "positive side" to bind to water molecules, and its negative charge to attract lithium ions.
Experiments have shown that the soft hydrogel battery absorbs only 19% of the moisture from the air at 50% humidity. This allows the battery to operate at a voltage of 3.1 V. Two notable drawbacks of the new battery are the faster loss of capacity - up to 60% of the initial level after 500 charging cycles (while modern batteries lose no more than 20%), as well as the low density of stored energy, amounting to only about 10% of the level of modern batteries.
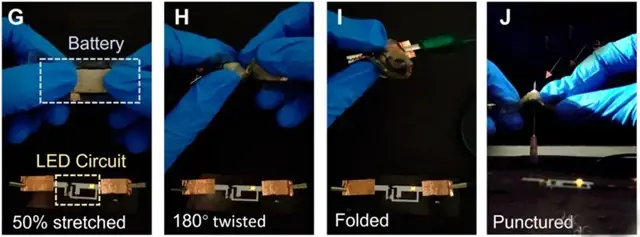
But the new soft battery in a flexible polymer package can be bent, twisted, punctured and even cut without consequences. It even recovers from "cuts" - although this requires it to be baked in a special chamber. Scientists are confident that the newly acquired properties will help create safer electronics - from robotics to wearable devices. In addition, the battery's characteristics can still be improved. It is only a matter of time and additional scientific research.
